Changing Our Coding: Genetic Editing
- Jia Chun
- Feb 21, 2025
- 4 min read

When I was younger, I wanted to be one of the PowerPuff girls. I dreamed of sprouting those huge eyes and donning a new hairstyle and a purple suit (is it obvious my favorite color was purple?) and joining the three other girls with my own purple streak extending behind me. I remember wishing I could somehow "edit" my DNA to have superpowers of my own and now, with the discovery of genetic editing, there might be a slim chance for my younger me's hopes and dreams (specifically, purple eyes).
As knowledge about the DNA (a tightly coiled strand that holds the information our bodies need to function) has increased, so has research into editing DNA. One such research project, published by Li et al. (this a special way researchers cite papers!), explores the advances in a genome editing technology called CRISPR.
ABSTRACT
Li et al. introduces the context of the experiment: CRISPR-based technology has been greatly researched upon, leading to new applications and formats. Li et al. summarizes advances of Cas orthologs (a technology extremely similar to CRISPR), new genome editing techniques, and discusses the applications of CRISPR-Cas in genome editing. The study also dives into the use of CRISPR for cardiovascular diseases (conditions effecting heart and blood vessels- for instance, heart attacks).
NOVEL CAS NUCLEASES
Now, in much easier, less scientific jargon terms, the true state of CRISPR works to fight of disease and virus in bacteria (here, viruses are our friends). Cas nucleases have dozens and dozens of subtypes, each with many different characteristics. Let's focus on Cas9 and Cas12a (for simplicity, let's call them #9 and #12), which have many great roles in the process of genome engineering.
Imagine the famous spiral of DNA anyone has been bound to have seen before. In the human body, DNA is microscopic, although if one cell's DNA is stretched to its true length, it would span 6 feet. In DNA are small coding elements called nucleotides (if familiar with coding, imagine nucleotides to be the 0s and 1s). Genetic diseases arise when the pattern of nucleotides has changed and this is the job CRISPR (in this case, #9 and #12 take on).
a: Deleting Large Number of Nucleotides
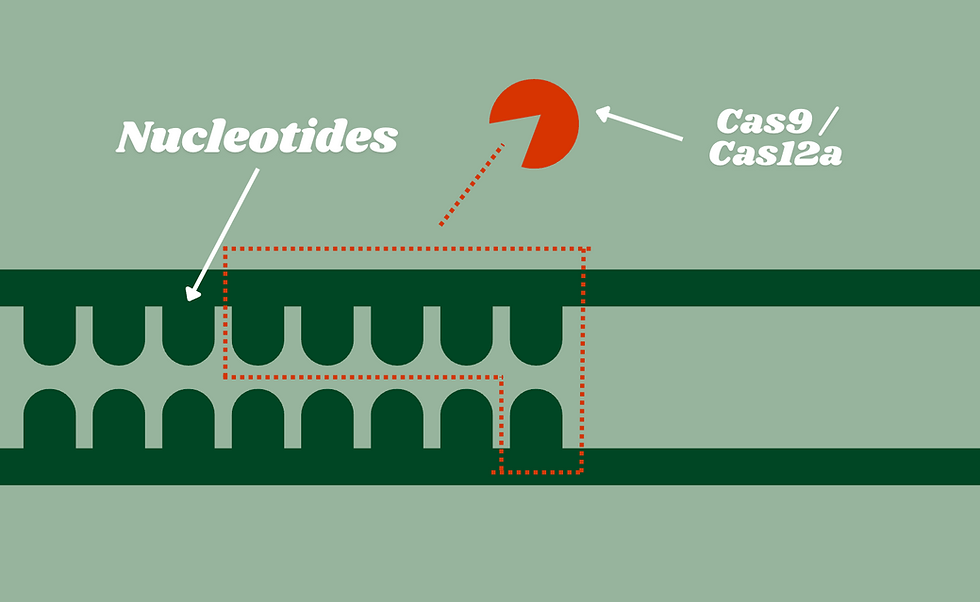
Novel Cas nucleases (including #9 and #12) have the capacity to delete many nucleotides. For example, Tay-Sachs disease stems from a gene with many mutated nucleotides, which could benefit from extracting many nucleotides, which increases efficiency in the process (although Tay-Sachs currently cannot be treated with genetic editing).
b: Miniature Sizes
To be able to maneuver into the cell's nucleus and effectively cut the DNA, Cas orthologs must be miniature. Having smaller variations of Cas helps scientists deliver it into the cell. For instance, a novel variant named IscB is two-fifths the size of Cas9 (to put this in perspective, Cas9 is invisible to the naked eye).
c: Diverse Recognition Capabilities
Specifically, in scientific terms, novel Cas variants have a diverse array of protospacer adjacent motif recognition capabilities. In much easier terms, new variants have the ability to recognize more binding sites (where they go on to cut the DNA). Genome editing needs a PAM sequence (a specific sequence of nucleotides) and Cas cannot go on to cut DNA without the existence of PAM.
Scientists attempted to create Cas nucleases that can bind to DNA without PAM sequences, but it may possibly reduce efficiency because Cas needs to look at the whole genome (which could take hours to a few days). So, scientists concluded that it would be much better to create PAM dependent Cas nucleases that could cover all genes. With this goal, scientists succeeded in creating three new variants that can read most PAM sequences (keep in mind that certain Cas nucleases are designed to read only one PAM sequence) and the variants have no preference. To summarize, scientists have discovered new variants that can detect where to bind quickly, making the process more efficient.
d: Improving Efficiency Without Affecting Cleavage Efficiency
In the world of genetic editing, off-target activity (when Cas nucleases target the wrong part of DNA or unintended genetic changes) is a major concern. Scientists explored the possibilities of increasing efficiency without changing cleavage (cutting) efficiency and succeeded in creating binding loops (simply makes it easier to bind to DNA) that can fix mismatches.
CONCLUSION
In conclusion, genome editing has been going through many, many improvements to be as efficient as possible. However, why is efficiency so crucial?
Genome editing is targeted towards fixing mutations in DNA (some commonly known genetic diseases are Sickle Cell Anemia, in which blood cells lose their circular shape). This has the huge potential to become a cure for previously incurable diseases, giving many hope.
As genetic editing must be extremely precise, as one single change or mistake in the process can lead to significant health consequences (in fact, these small changes in DNA - even as small as one nucleotide change in trillions of nucleotides- cause these diseases). In order to be successful, technology must be as efficient as possible. And as scientists may continue to provide this treatment for those with genetic diseases, success must be seen as the thin line between life and death, since they are dealing with human lives.
On a higher note, as shown in this simplified research paper, efficiency has improved quite a bit since CRISPR was initially discovered and there are a cases in which patients had their lives changed.
Mini-Dictionary
CRISPR - Clustered Regularly Interspaced Short Palindromic Repeats (can be programmed to edit DNA)
Cas nucleases - Proteins that enter the cell and cut DNA

Comments